Theme: Novel Strategies and Emerging Technologies of Biotech & Biomedical industries
Pharma Biotech Expo 2019
- About Conference
- Sessions/ Tracks
- Market Analysis
- Past Conference
- Keynote Speakers
- Chairs/Co-Chairs
- Moderators
- Sponsors/Exhibitors
- Media Partners
World Congress on Pharmaceutical Biotechnology and Bioengineering Conference is scheduled to be held during March 25-26, at Orlando, USA. This Pharma Biotech & Biomed includes a wide range of Keynote presentations, plenary talks, Symposia, Workshops, Exhibitions, Poster presentations and Career development programs with an audience from various areas of Pharma Biotech and Biomedical assembling experts from Academy and Research-Professors, Assistant Professors, PhD Scholars, Pharmaceutical Associations and Societies, Pharmaceutical Business Entrepreneurs, Pharmaceutical Manufacturing Companies, Medical Devices Manufacturing Companies, Directors, CEO’s of Organizations, CROs and Clinical Data Management Companies.
This meeting focuses on the trends which the biotechnology and Biomedical market is heading on since its launch till the present date and moving on to the projections in future. It takes into its fold the critical developmental procedures, analyses, regulatory factors including post marketing surveillance and clinical studies. Innovation and compliance to regulatory guidelines are the most critical aspects.
Conference series LLC Ltd Organizes 3000+ Global Events Every Year across USA, Europe & Asia with support from 1000 more scientific societies and Publishes 700+ Open access journals which contains over 100000 eminent personalities, reputed scientists as editorial board and organizing committee members. The conference series LLC Ltd website will provide you list and details about the conferences organize worldwide.
Details About Pharmaceutical Biotechnology & Bioengineering Conference in 2019 in USA:
ConferenceSeries.com organizing Pharmaceutical Sciences Conferences in 2019 in USA. We organize Pharmacology Meetings in the fields related to Pharmaceutical Sciences.
| Conference Name | Place | Date |
|---|---|---|
| Pharmaceutical Sciences Conferences 2019: Bioengineering & Biotechnology | Orlando, Florida, USA | March 25-26, 2019 |
Why to Attend?
On the basis of application, the global biotechnology market is broadly categorized into bioagriculture, biopharmacy, bioindustrial, and bioservices. In 2011, the biopharmaceuticals segment accounted for a 60% share in the market owing to an increase in government funding, widespread technological advancements, and availability of cheap labor in developing economies such as India, China, and Thailand. Increasing demand for food in developing countries is expected to boost the market. Bioseeds offer greater advantages compared to conventional seeds and hence will register exponential growth in demand during the forecast period. This will propel the growth of the bioagriculture segment. By 2018, the segment is projected to reach a valuation of US$27.46 bn.
Join your peers around the world focused on learning about Biotechnology, Biomedical,Biologics and Biosimilars related advances, which is your single best opportunity to reach the largest assemblage of participants from the Biotechnolgy community, conduct demonstrations, distribute information, meet with current and potential professionals, make a splash with a new research works, and receive name recognition at this 2-day event. World-renowned speakers, the most recent research, advances, and the newest updates in Biotech and Biomedical are hallmarks of this conference.
Who Should Attend and Who You’ll Meet:
• Pharmaceutical, Biotech, Biomedical, Industry CEO, Directors, R&D Researchers
• Business Entrepreneurs, Training Institutes, Software creating organizations,
• Medical Devices Manufacturing Companies, CRO
• Data Management, Clinical Trial Conducting Organizations and Companies, Association and Societies
• Students, Scientists, Researchers, and Faculty of Pharmaceutical Sciences
• Universities, Medical Colleges, Researchers from Pharmaceutical foundation
On behalf of Organizing committee members, we invites all the participants from all over the world to attend World Congress on Pharmaceutical Biotechnology and Bioengineering during March 25-27, 2019, Orlando, Florida, USA which includes prompt Keynote presentations, Oral talks, Poster presentations and Exhibitions.
Pharma Biotech Expo 2019offers a best platform with its well organized scientific program to the audience which includes interactive panel discussions, keynote lectures, plenary talks and poster sessions on the topics Biotechnology in Drug Discovery and Development, Pharmaceutical Biotechnology: Concepts and Applications, Biopharmaceuticals and Biotheruapeutics: Recent Advancements, Pharmaceutical Innovation in the 21st Century, new scientific approaches to international regulatory standards. Biotechnology Companies & Market Analysis, The conference invites delegates from Biotech Company’s, Bioengineer’s, Academicians, Clinicians, Researchers, Health care professionals, students, business delegates and Young researchers across the globe providing a better podium, interconnecting the latest research, technological developments in the arena as well as therapeutic aspects.
Track 1: Biotechnology in Drug Discovery and Development
The Pharma industry is still assimilating the late 1990s wave of drug discovery technologies that are bringing about a powerful convergence of molecular biology, miniaturization, and materials and information technologies. Computer and chip technologies have given thousands of scientists the human genome as a bench tool, an opportunity unthinkable just 10 years ago. Scientists already have a publicly available catalog of 1.8 million single nucleotide polymorphisms to work with, and geometric expansion of proteomics research and technologies will create multiples of the existing data volume. The convergence of information technology and biology may well be the biggest story in biotechnology over the next decade. As we learn more about the cascades of reactions essential to disease and health, in silico modeling and testing will become more refined, perhaps alleviating some of the bottlenecks characteristic of wet biology.
Related Biotechnology Conferences | Bioengineering Meetings
BIO Investor Forum October 17 – October 18, 2018 San Francisco, California | BIO Patient and Health Advocacy Summit October 30 – October 31, 2018 Washington, DC | ICCA activities at IMEX America 2018 October 16 - 18 October 2018 Las Vegas, NV, U.S.A | ICCA activities at IMEX 2019 May 21 - 23 May 2019 | 1st Association of Southeast Asian Prosthodontic Societies and 7th Malaysian Association for Prosthodontic Scientific Conference 5 - 8 Oct 2018 Kuala Lumpur | EARCOS Leadership Conference (ELC) 2018, 25 - 27 Oct 2018, Kuala Lumpur | Annual Conference of the International Federation of Technical Analysts (IFTA) 2018 26 - 28 Oct 2018, Kuala Lumpur | Passive and Low Energy Architecture (PLEA) 2018: Smart and Healthy within the 2-degree Limit Dec 10 - Dec 12 2018, | 17th Asia Pacific Life Insurance Congress, May 30-Jun 01 2019.
Related Associations and Societies: International Federation for Medical and Biological Engineering | European Society for Biomaterials (ESB) | Biomedical Engineering Society (BMES) | American Institute for Medical and Biological Engineering | The European Association of Pharma Biotechnology (EAPB) | The Biotech Research Society | Young European Biotech Network (YEBN) | Japan Bioindustry Association (JBA) | European Federation of Pharmaceutical Industries and Associations (EFPIA)
Track 2: Pharmaceutical Biotechnology: Concepts and Applications
The global biotechnology market is expected to reach USD 727.1 billion by 2025, according to a new report by Grand View Research, Inc. The emergence of certain key themes in the biotechnology market is expected to drive growth in this industry to a lucrative extent.
These key themes include regenerative medicine and genetics in diagnostics. The companies focusing on the development of regenerative therapies is anticipated to drive sector growth through to 2025. Technological advancements pertaining to the penetration of artificial intelligence in this industry is expected to fuel progress with potential avenues. The companies are engaged in unleashing machine learning in order to understand individual cancer cases, while recommending clinical trials.
Supportive government (and its allied agencies) policies related to synthetic biology is a major growth impacting driver in this sector. Developed economies such as UK and the U.S. are critically monitoring and funding synthetic biology R&D initiatives. For example, in 2010, a six months’ review of synthetic biology headed by a panel of expert scientists was enforced by the U.S. President and subsequently conducted a hearing of the Energy and Commerce Committee exclusively concerning synthetic biology.
Related Biotechnology Conferences | Bioengineering Meetings
BIO Investor Forum October 17 – October 18, 2018 San Francisco, California | BIO Patient and Health Advocacy Summit October 30 – October 31, 2018 Washington, DC | ICCA activities at IMEX America 2018 October 16 - 18 October 2018 Las Vegas, NV, U.S.A | ICCA activities at IMEX 2019 May 21 - 23 May 2019 | 1st Association of Southeast Asian Prosthodontic Societies and 7th Malaysian Association for Prosthodontic Scientific Conference 5 - 8 Oct 2018 Kuala Lumpur | EARCOS Leadership Conference (ELC) 2018, 25 - 27 Oct 2018, Kuala Lumpur | Annual Conference of the International Federation of Technical Analysts (IFTA) 2018 26 - 28 Oct 2018, Kuala Lumpur | Passive and Low Energy Architecture (PLEA) 2018: Smart and Healthy within the 2-degree Limit Dec 10 - Dec 12 2018, | 17th Asia Pacific Life Insurance Congress, May 30-Jun 01 2019.
Related Associations and Societies: International Federation for Medical and Biological Engineering | European Society for Biomaterials (ESB) | Biomedical Engineering Society (BMES) | American Institute for Medical and Biological Engineering | The European Association of Pharma Biotechnology (EAPB) | The Biotech Research Society | Young European Biotech Network (YEBN) | Japan Bioindustry Association (JBA) | European Federation of Pharmaceutical Industries and Associations (EFPIA)
Track 3: Biopharmaceuticals and Biotherapeutics: Recent Advancements
Over the past two decades there has been a decisive shift towards large molecule, biological drugs or biopharmaceuticals, and away from traditional chemically synthesised, small molecule drugs Revenue from biopharmaceuticals in 2016 was in the range of $280-300 billion and is forecasted to grow at about 10% for the foreseeable future. One particular biomolecule dominates though in biopharmaceuticals currently and that is the monoclonal antibody.
The number approved for use as biopharmaceuticals has steadily increased and in 2016 accounted for five of the top ten selling drugs globally with revenues of $46 billion
This appetite for monoclonal drugs shows no sign of abating with this article on the therapeutic monoclonal antibody market anticipating 2020 revenues of $125 billion. A recent FiercePharma report also predicted that by 2022 monoclonal antibodies will make up 60% of the top selling cancer drugs.
A biologic therapeutic product, also known as a biologic, is a therapeutic product developed to treat a variety of diseases. This biologic product can be a monoclonal antibody, a vaccine, a tissue, or various proteins such as cytokines, enzymes, fusion proteins, whole cells, and viral and nonviral gene therapies
Related Biotechnology Conferences | Bioengineering Meetings
BIO Investor Forum October 17 – October 18, 2018 San Francisco, California | BIO Patient and Health Advocacy Summit October 30 – October 31, 2018 Washington, DC | ICCA activities at IMEX America 2018 October 16 - 18 October 2018 Las Vegas, NV, U.S.A | ICCA activities at IMEX 2019 May 21 - 23 May 2019 | 1st Association of Southeast Asian Prosthodontic Societies and 7th Malaysian Association for Prosthodontic Scientific Conference 5 - 8 Oct 2018 Kuala Lumpur | EARCOS Leadership Conference (ELC) 2018, 25 - 27 Oct 2018, Kuala Lumpur | Annual Conference of the International Federation of Technical Analysts (IFTA) 2018 26 - 28 Oct 2018, Kuala Lumpur | Passive and Low Energy Architecture (PLEA) 2018: Smart and Healthy within the 2-degree Limit Dec 10 - Dec 12 2018, | 17th Asia Pacific Life Insurance Congress, May 30-Jun 01 2019.
Related Associations or Societies: International Federation for Medical and Biological Engineering | European Society for Biomaterials (ESB) | Biomedical Engineering Society (BMES) | American Institute for Medical and Biological Engineering | The European Association of Pharma Biotechnology (EAPB) | The Biotech Research Society | Young European Biotech Network (YEBN) | Japan Bioindustry Association (JBA) | European Federation of Pharmaceutical Industries and Associations (EFPIA)
Track 4: Biotech Products Manufacturing: Novel Approaches & Technologies
The incredible amount of recent biologic therapeutic discoveries has led to an increased need for scientific and engineering knowledge available to characterize and bio manufacture these large and complex molecules. Today’s biologic manufacturing facilities incorporate analytical and process development capabilities to develop and test the scale-up of the process to deliver sufficient productivity of a quality product.
There are various regulatory opinions in the United States, European, Japanese pharmaceutical industries, and other countries that regulate biologics.
Related Biotechnology Conferences | Bioengineering Meetings
BIO Investor Forum October 17 – October 18, 2018 San Francisco, California | BIO Patient and Health Advocacy Summit October 30 – October 31, 2018 Washington, DC | ICCA activities at IMEX America 2018 October 16 - 18 October 2018 Las Vegas, NV, U.S.A | ICCA activities at IMEX 2019 May 21 - 23 May 2019 | 1st Association of Southeast Asian Prosthodontic Societies and 7th Malaysian Association for Prosthodontic Scientific Conference 5 - 8 Oct 2018 Kuala Lumpur | EARCOS Leadership Conference (ELC) 2018, 25 - 27 Oct 2018, Kuala Lumpur | Annual Conference of the International Federation of Technical Analysts (IFTA) 2018 26 - 28 Oct 2018, Kuala Lumpur | Passive and Low Energy Architecture (PLEA) 2018: Smart and Healthy within the 2-degree Limit Dec 10 - Dec 12 2018, | 17th Asia Pacific Life Insurance Congress, May 30-Jun 01 2019.
Related Associations or Societies: International Federation for Medical and Biological Engineering | European Society for Biomaterials (ESB) | Biomedical Engineering Society (BMES) | American Institute for Medical and Biological Engineering | The European Association of Pharma Biotechnology (EAPB) | The Biotech Research Society | Young European Biotech Network (YEBN) | Japan Bioindustry Association (JBA) | European Federation of Pharmaceutical Industries and Associations (EFPIA)
Track 5: Biopharmaceutical Production in Transgenic Animals
A decade of success in expressing a variety of proteins in livestock has brought three human recombinant proteins to human clinical trials. Recent progress has drawn on molecular biology and reproductive physiology to improve the efficiency of producing and reproducing useful transgenic founder animals, and to improve the expression of heterologous proteins.
The market growth has been the driving force on efforts for the development of new therapeutic proteins, in which transgenesis emerges as key component. The use of the transgenic animal platform offers attractive possibilities, residing on the low production costs allied to high productivity and quality of the recombinant proteins
The approval of two mammary gland-derived recombinant proteins for commercial and clinical use has boosted the interest for more efficient, safer and economic ways to generate transgenic founders to meet the increasing demand for biomedical proteins worldwide.
Related Biotechnology Conferences | Bioengineering Meetings
BIO Investor Forum October 17 – October 18, 2018 San Francisco, California | BIO Patient and Health Advocacy Summit October 30 – October 31, 2018 Washington, DC | ICCA activities at IMEX America 2018 October 16 - 18 October 2018 Las Vegas, NV, U.S.A | ICCA activities at IMEX 2019 May 21 - 23 May 2019 | 1st Association of Southeast Asian Prosthodontic Societies and 7th Malaysian Association for Prosthodontic Scientific Conference 5 - 8 Oct 2018 Kuala Lumpur | EARCOS Leadership Conference (ELC) 2018, 25 - 27 Oct 2018, Kuala Lumpur | Annual Conference of the International Federation of Technical Analysts (IFTA) 2018 26 - 28 Oct 2018, Kuala Lumpur | Passive and Low Energy Architecture (PLEA) 2018: Smart and Healthy within the 2-degree Limit Dec 10 - Dec 12 2018, | 17th Asia Pacific Life Insurance Congress, May 30-Jun 01 2019.
Related Associations or Societies: International Federation for Medical and Biological Engineering | European Society for Biomaterials (ESB) | Biomedical Engineering Society (BMES) | American Institute for Medical and Biological Engineering | The European Association of Pharma Biotechnology (EAPB) | The Biotech Research Society | Young European Biotech Network (YEBN) | Japan Bioindustry Association (JBA) | European Federation of Pharmaceutical Industries and Associations (EFPIA)
Track 6: Nanotechnology in Biopharmaceuticals
The application of nanobiotechnology has far ranging uses within a number of sectors of the life sciences industry, including drug discovery, formulation, clinical assessment, drug delivery and monitoring. The reasons for the increase in value was said to be multifaceted, relating to developments in cancer and viral treatments consolidation in the pharmaceutical industry and the expiration of patents. Pressure on companies to prolong the lifecycle of existing drugs and also to produce new products is a driver behind the increased use of biopharmaceutical nanotechnology
Related Biotechnology Conferences | Bioengineering Meetings
BIO Investor Forum October 17 – October 18, 2018 San Francisco, California | BIO Patient and Health Advocacy Summit October 30 – October 31, 2018 Washington, DC | ICCA activities at IMEX America 2018 October 16 - 18 October 2018 Las Vegas, NV, U.S.A | ICCA activities at IMEX 2019 May 21 - 23 May 2019 | 1st Association of Southeast Asian Prosthodontic Societies and 7th Malaysian Association for Prosthodontic Scientific Conference 5 - 8 Oct 2018 Kuala Lumpur | EARCOS Leadership Conference (ELC) 2018, 25 - 27 Oct 2018, Kuala Lumpur | Annual Conference of the International Federation of Technical Analysts (IFTA) 2018 26 - 28 Oct 2018, Kuala Lumpur | Passive and Low Energy Architecture (PLEA) 2018: Smart and Healthy within the 2-degree Limit Dec 10 - Dec 12 2018, | 17th Asia Pacific Life Insurance Congress, May 30-Jun 01 2019.
Related Associations or Societies: International Federation for Medical and Biological Engineering | European Society for Biomaterials (ESB) | Biomedical Engineering Society (BMES) | American Institute for Medical and Biological Engineering | The European Association of Pharma Biotechnology (EAPB) | The Biotech Research Society | Young European Biotech Network (YEBN) | Japan Bioindustry Association (JBA) | European Federation of Pharmaceutical Industries and Associations (EFPIA)
Track 7: Pharmacogenomics & Pharmacogenetics
With the advancement of human genome science now it will be easy for our clinicians to tailor the drug treatment through the specific prescription to the individual patient that target the drug to maximize its therapeutic efficacy and minimize the damage to surrounding healthy cells. The clinical trial studies can be adopted the advanced validated pharmacogenetic markers in order to increase the demonstration of therapeutic benefits without exposing non-receptive subjects. The clinical trial study can also be optimized as a small, fast and economic by undertaking pregenetic screening of those patients taking part in a clinical trial.
Global Pharmacogenomics Market Analysis: Focus on Ecosystem Players (Diagnostic Test, Pharmaceutical & Others), Therapeutic Applications (Oncology, Cardiovascular & Others)
The global Pharmacogenomics market is expected to grow over $14.85 billion by 2022. Rapid advancement and innovation of new healthcare technologies such as next generation sequencing, High Throughput Screening, and Digital Polymerase Chain Reaction are developing a strong base for the growth of Pharmacogenomics market
Related Biotechnology Conferences | Bioengineering Meetings
BIO Investor Forum October 17 – October 18, 2018 San Francisco, California | BIO Patient and Health Advocacy Summit October 30 – October 31, 2018 Washington, DC | ICCA activities at IMEX America 2018 October 16 - 18 October 2018 Las Vegas, NV, U.S.A | ICCA activities at IMEX 2019 May 21 - 23 May 2019 | 1st Association of Southeast Asian Prosthodontic Societies and 7th Malaysian Association for Prosthodontic Scientific Conference 5 - 8 Oct 2018 Kuala Lumpur | EARCOS Leadership Conference (ELC) 2018, 25 - 27 Oct 2018, Kuala Lumpur | Annual Conference of the International Federation of Technical Analysts (IFTA) 2018 26 - 28 Oct 2018, Kuala Lumpur | Passive and Low Energy Architecture (PLEA) 2018: Smart and Healthy within the 2-degree Limit Dec 10 - Dec 12 2018, | 17th Asia Pacific Life Insurance Congress, May 30-Jun 01 2019.
Related Associations or Societies: International Federation for Medical and Biological Engineering | European Society for Biomaterials (ESB) | Biomedical Engineering Society (BMES) | American Institute for Medical and Biological Engineering | The European Association of Pharma Biotechnology (EAPB) | The Biotech Research Society | Young European Biotech Network (YEBN) | Japan Bioindustry Association (JBA) | European Federation of Pharmaceutical Industries and Associations (EFPIA)
Track 8: Vaccines Manufacturing: Challenges and Solutions
The biotechnology era has experienced significant changes in the number of companies involved in vaccine manufacturing as well as in the production systems they use. Nevertheless, challenges in this area are multiple. In the current vaccine-manufacturing environment, time to market and cost effectiveness are key issues that need to be addressed in addition to smooth R&D and clinical studies. Furthermore, scale up and safety are important for maintaining a successful manufacturing process. As a result, state-of-the-art technologies to simplify vaccine development and manufacturing are becoming ever-more crucial
Vaccine development was discussed as important for global security at the World Economic Forum in Davos, Switzerland in January 2015. The first draft of the US House of Representatives “21st Century Cures” initiative includes actions to speed up vaccine approval and coverage. Proposals seek to tighten timelines for the Centers for Disease Control and Prevention (CDC) to recommend new vaccines, for FDA to expedite vaccine exports, and for the National Institutes of Health (NIH) to advance vaccine development. Researchers are looking for formulations that reduce cold-chain requirements, methods to boost process yield, and new approaches to document product quality.
The recent influenza vaccine shortages have provided a timely reminder of the tenuous nature of the world's vaccine supply and the potential for manufacturing issues to severely disrupt vital access to important vaccines. The application of new technologies to the discovery, assessment, development and production of vaccines has the potential to prevent such occurrences and enable the introduction of new vaccines. Gene-based vaccines, virus-like particles, plant-derived vaccines and novel adjuvants and delivery systems represent promising approaches to creating safer, more potent vaccines. As a consequence, more people will have faster access to more effective vaccines against a broader spectrum of infectious diseases. However, the increased cost of producing new vaccines and regulatory uncertainty remain challenges for vaccine manufacturers.
Related Biotechnology Conferences | Bioengineering Meetings
BIO Investor Forum October 17 – October 18, 2018 San Francisco, California | BIO Patient and Health Advocacy Summit October 30 – October 31, 2018 Washington, DC | ICCA activities at IMEX America 2018 October 16 - 18 October 2018 Las Vegas, NV, U.S.A | ICCA activities at IMEX 2019 May 21 - 23 May 2019 | 1st Association of Southeast Asian Prosthodontic Societies and 7th Malaysian Association for Prosthodontic Scientific Conference 5 - 8 Oct 2018 Kuala Lumpur | EARCOS Leadership Conference (ELC) 2018, 25 - 27 Oct 2018, Kuala Lumpur | Annual Conference of the International Federation of Technical Analysts (IFTA) 2018 26 - 28 Oct 2018, Kuala Lumpur | Passive and Low Energy Architecture (PLEA) 2018: Smart and Healthy within the 2-degree Limit Dec 10 - Dec 12 2018, | 17th Asia Pacific Life Insurance Congress, May 30-Jun 01 2019.
Related Associations or Societies: International Federation for Medical and Biological Engineering | European Society for Biomaterials (ESB) | Biomedical Engineering Society (BMES) | American Institute for Medical and Biological Engineering | The European Association of Pharma Biotechnology (EAPB) | The Biotech Research Society | Young European Biotech Network (YEBN) | Japan Bioindustry Association (JBA) | European Federation of Pharmaceutical Industries and Associations (EFPIA)
Track 9: Recombinant DNA Technology in Pharma and Medicine
The recombinant production of therapeutic proteins for human diseases is currently the largest source of innovation in the pharmaceutical industry
The global recombinant DNA technology market is expected to reach USD 844.6 billion by 2025, according to a new report by Grand View Research, Inc. Success of genetically engineered human insulin in diabetes treatment has triggered the development of many other recombinant therapeutics and drugs. This has translated to the huge success of Recombinant DNA technology. This technology has offered significant prospects for elucidating the gap between disease and its effective treatment.
Widespread successful application of this technique in veterinary product development, genetically modified crop development, bio pesticides & biofuel production, and gene therapy, are expected to spur the adoption of this technology throughout the forecast period. Use of Genetically Modified products, such as Genetically Modified animals, developed using rDNA method are found to be indispensable to accelerate medical research. Furthermore, more than 3000 scientific studies have been carried out to assess the GM products safety in context to its impact on human health and environment.
According to Statistics MRC, the Global Recombinant DNA Technology Market is accounted for $499.8 million in 2016 and expected to grow at a CAGR of 6.9% to reach $799.9 million by 2023.
Related Biotechnology Conferences | Bioengineering Meetings
BIO Investor Forum October 17 – October 18, 2018 San Francisco, California | BIO Patient and Health Advocacy Summit October 30 – October 31, 2018 Washington, DC | ICCA activities at IMEX America 2018 October 16 - 18 October 2018 Las Vegas, NV, U.S.A | ICCA activities at IMEX 2019 May 21 - 23 May 2019 | 1st Association of Southeast Asian Prosthodontic Societies and 7th Malaysian Association for Prosthodontic Scientific Conference 5 - 8 Oct 2018 Kuala Lumpur | EARCOS Leadership Conference (ELC) 2018, 25 - 27 Oct 2018, Kuala Lumpur | Annual Conference of the International Federation of Technical Analysts (IFTA) 2018 26 - 28 Oct 2018, Kuala Lumpur | Passive and Low Energy Architecture (PLEA) 2018: Smart and Healthy within the 2-degree Limit Dec 10 - Dec 12 2018, | 17th Asia Pacific Life Insurance Congress, May 30-Jun 01 2019.
Related Associations or Societies: International Federation for Medical and Biological Engineering | European Society for Biomaterials (ESB) | Biomedical Engineering Society (BMES) | American Institute for Medical and Biological Engineering | The European Association of Pharma Biotechnology (EAPB) | The Biotech Research Society | Young European Biotech Network (YEBN) | Japan Bioindustry Association (JBA) | European Federation of Pharmaceutical Industries and Associations (EFPIA)
Track 10: Bioavailability, Bioequivalence & Bioethics
The concept of BE has been accepted worldwide by the pharmaceutical industry and national regulatory authorities for over 20 years and is applied to new as well as generic products. As a result, thousands of high-quality generic drugs at reduced costs have become available in every corner of the globe. The assessment of BE is not a simple issue, however, and much of the research has been done in recent years to develop new and more effective approaches to the assessment of BE.
Since then, and after turn of the century, tremendous advancements have been made by the FDA and other regulatory authorities (national, international, and supranational), and by industry and academia in the area of assessment of bioequivalence. Currently approaches to determine BE of pharmaceutical products has been largely standardized. This has occurred due to discussion and consensus reached among various stakeholders at numerous national and international meetings, conferences, and workshops.
The pharmaceutical firms all over the world are burning the candle at both ends for attainment of market authorizations in various nations.
Bioethics can evaluate global justice by weighing human rights theory and future innovation at the macro level, and by addressing market forces and responsibilities at the micro level. Inherent structural features of pharmaceuticals, such as its reliance on research and development, cause the industry to employ pricing strategies that seem counter-intuitive to conventional wisdom, but that result in producing a just allocation as defined by market forces.
Related Biotechnology Conferences | Bioengineering Meetings
BIO Investor Forum October 17 – October 18, 2018 San Francisco, California | BIO Patient and Health Advocacy Summit October 30 – October 31, 2018 Washington, DC | ICCA activities at IMEX America 2018 October 16 - 18 October 2018 Las Vegas, NV, U.S.A | ICCA activities at IMEX 2019 May 21 - 23 May 2019 | 1st Association of Southeast Asian Prosthodontic Societies and 7th Malaysian Association for Prosthodontic Scientific Conference 5 - 8 Oct 2018 Kuala Lumpur | EARCOS Leadership Conference (ELC) 2018, 25 - 27 Oct 2018, Kuala Lumpur | Annual Conference of the International Federation of Technical Analysts (IFTA) 2018 26 - 28 Oct 2018, Kuala Lumpur | Passive and Low Energy Architecture (PLEA) 2018: Smart and Healthy within the 2-degree Limit Dec 10 - Dec 12 2018, | 17th Asia Pacific Life Insurance Congress, May 30-Jun 01 2019.
Related Associations or Societies: International Federation for Medical and Biological Engineering | European Society for Biomaterials (ESB) | Biomedical Engineering Society (BMES) | American Institute for Medical and Biological Engineering | The European Association of Pharma Biotechnology (EAPB) | The Biotech Research Society | Young European Biotech Network (YEBN) | Japan Bioindustry Association (JBA) | European Federation of Pharmaceutical Industries and Associations (EFPIA)
Track 11: Biomedical Engineering in Healthcare
It is a fast-growing area of engineering and individuals would typically find themselves working in health services, the medical devices industry or research.
Scientists, researchers, and manufacturers in the medical and pharmaceutical sectors will continue to call upon biomedical engineers to address injuries and physical disabilities; to develop advanced prostheses and artificial body organs; and to improve healthcare technology and rehabilitation practices. As baby boomers live longer and remain active, there will be increased demand for biomedical devices and procedures, such as hip and knee replacements.
When surveying the sector, it is also important to factor in the growing digital healthcare market. This is expected to be worth $233.3bn by 2020 (up from $60.8bn in 2013), with Asia-Pacific predicted as a key region.
Related Biotechnology Conferences | Bioengineering Meetings
BIO Investor Forum October 17 – October 18, 2018 San Francisco, California | BIO Patient and Health Advocacy Summit October 30 – October 31, 2018 Washington, DC | ICCA activities at IMEX America 2018 October 16 - 18 October 2018 Las Vegas, NV, U.S.A | ICCA activities at IMEX 2019 May 21 - 23 May 2019 | 1st Association of Southeast Asian Prosthodontic Societies and 7th Malaysian Association for Prosthodontic Scientific Conference 5 - 8 Oct 2018 Kuala Lumpur | EARCOS Leadership Conference (ELC) 2018, 25 - 27 Oct 2018, Kuala Lumpur | Annual Conference of the International Federation of Technical Analysts (IFTA) 2018 26 - 28 Oct 2018, Kuala Lumpur | Passive and Low Energy Architecture (PLEA) 2018: Smart and Healthy within the 2-degree Limit Dec 10 - Dec 12 2018, | 17th Asia Pacific Life Insurance Congress, May 30-Jun 01 2019.
Related Associations or Societies: International Federation for Medical and Biological Engineering | European Society for Biomaterials (ESB) | Biomedical Engineering Society (BMES) | American Institute for Medical and Biological Engineering | The European Association of Pharma Biotechnology (EAPB) | The Biotech Research Society | Young European Biotech Network (YEBN) | Japan Bioindustry Association (JBA) | European Federation of Pharmaceutical Industries and Associations (EFPIA)
Track 12: Recent Advances in Diagnostic and Therapeutic Systems
With several medical conditions requiring extensive and continuous monitoring and early and accurate diagnosis becoming increasingly desirable, technology for biomedical applications is rapidly becoming one of the key ingredients of today and tomorrow’s medical care.
Diagnostic Test segment is holding the maximum market share and is expected to grow at 11.63% CAGR from 2016 to 2022. This is due to the increasing demand for diagnostic tests as patients are emphasizing more on pre-diagnosis treatments, increasing genetic disorders & mutational diseases, and government initiatives and investment in research and development programs among others.
Over the past two decades a vast new armamentarium of diagnostic techniques has revolutionized the practice of medicine. The entire human body can now be imaged in exquisite anatomical detail. Computed tomography (CT), magnetic resonance imaging (MRI), and ultrasonography routinely “section” patients into slices less than a centimeter thick. Abnormalities can be detected well before they produce any clinical signs or symptoms. Undoubtedly, these technological advances have enhanced the physician's potential for understanding disease and treating patients.
Related Biotechnology Conferences | Bioengineering Meetings
BIO Investor Forum October 17 – October 18, 2018 San Francisco, California | BIO Patient and Health Advocacy Summit October 30 – October 31, 2018 Washington, DC | ICCA activities at IMEX America 2018 October 16 - 18 October 2018 Las Vegas, NV, U.S.A | ICCA activities at IMEX 2019 May 21 - 23 May 2019 | 1st Association of Southeast Asian Prosthodontic Societies and 7th Malaysian Association for Prosthodontic Scientific Conference 5 - 8 Oct 2018 Kuala Lumpur | EARCOS Leadership Conference (ELC) 2018, 25 - 27 Oct 2018, Kuala Lumpur | Annual Conference of the International Federation of Technical Analysts (IFTA) 2018 26 - 28 Oct 2018, Kuala Lumpur | Passive and Low Energy Architecture (PLEA) 2018: Smart and Healthy within the 2-degree Limit Dec 10 - Dec 12 2018, | 17th Asia Pacific Life Insurance Congress, May 30-Jun 01 2019.
Related Associations or Societies: International Federation for Medical and Biological Engineering | European Society for Biomaterials (ESB) | Biomedical Engineering Society (BMES) | American Institute for Medical and Biological Engineering | The European Association of Pharma Biotechnology (EAPB) | The Biotech Research Society | Young European Biotech Network (YEBN) | Japan Bioindustry Association (JBA) | European Federation of Pharmaceutical Industries and Associations (EFPIA)
Track 13: Medical Devices: Challenges and Applications
From miniaturized home diagnostic instruments to therapeutic devices and to large scale hospital imaging and monitoring systems, healthcare is becoming increasingly dependent on technology. This course meets the growing need for biomedical and clinical engineers across the world by focusing on the design of medical devices from conception to application.
One of the few accredited courses of its kind in London, the program concentrates on the use of biomedical-driven engineering design and technology in healthcare settings so you can approach this multidisciplinary topic from the biological and medical perspective; the technological design and development perspective; and from the perspective of managing the organisation and maintenance of large scale equipment and IT systems in a hospital.
While its fragmented nature makes it difficult to quantify the overall biomedical engineering sector in terms of number of people employed and its worth, the global medical devices market alone is estimated to be worth US$381bn, according to medical market research company Kalorama.
Related Biotechnology Conferences | Bioengineering Meetings
BIO Investor Forum October 17 – October 18, 2018 San Francisco, California | BIO Patient and Health Advocacy Summit October 30 – October 31, 2018 Washington, DC | ICCA activities at IMEX America 2018 October 16 - 18 October 2018 Las Vegas, NV, U.S.A | ICCA activities at IMEX 2019 May 21 - 23 May 2019 | 1st Association of Southeast Asian Prosthodontic Societies and 7th Malaysian Association for Prosthodontic Scientific Conference 5 - 8 Oct 2018 Kuala Lumpur | EARCOS Leadership Conference (ELC) 2018, 25 - 27 Oct 2018, Kuala Lumpur | Annual Conference of the International Federation of Technical Analysts (IFTA) 2018 26 - 28 Oct 2018, Kuala Lumpur | Passive and Low Energy Architecture (PLEA) 2018: Smart and Healthy within the 2-degree Limit Dec 10 - Dec 12 2018, | 17th Asia Pacific Life Insurance Congress, May 30-Jun 01 2019.
Related Associations or Societies: International Federation for Medical and Biological Engineering | European Society for Biomaterials (ESB) | Biomedical Engineering Society (BMES) | American Institute for Medical and Biological Engineering | The European Association of Pharma Biotechnology (EAPB) | The Biotech Research Society | Young European Biotech Network (YEBN) | Japan Bioindustry Association (JBA) | European Federation of Pharmaceutical Industries and Associations (EFPIA)
Track 14: Pharmaceutical & Genetic Engineering
Modern pharmaceutical biotechnology encompasses gene cloning and recombinant DNA technology. The prime focus of the study was to know in depth of the concepts of Genetic engineering that are implemented in pharmaceutical industry. This study unleashes the paramount significance of Genetic engineering emerged out with outstanding success. Pharmaceutical trends are moving towards two profound technologies the first being Novel Drug Delivery system. This review work ensures the concepts of Recombinant rDNA, and the hybridoma techniques that are implemented in drug discovery and research. Genetic engineering now allows biological synthesis and large-scale production of several proteins with therapeutic potential. The principal challenge in this sphere is to identify new, medically and commercially significant targets. In the future, genetic engineering will surely provide invaluable tools for the study of the molecular basis of cellular control and pathophysiology, which will permit biochemists and medicinal chemists to design novel medicines.
Related Biotechnology Conferences | Bioengineering Meetings
BIO Investor Forum October 17 – October 18, 2018 San Francisco, California | BIO Patient and Health Advocacy Summit October 30 – October 31, 2018 Washington, DC | ICCA activities at IMEX America 2018 October 16 - 18 October 2018 Las Vegas, NV, U.S.A | ICCA activities at IMEX 2019 May 21 - 23 May 2019 | 1st Association of Southeast Asian Prosthodontic Societies and 7th Malaysian Association for Prosthodontic Scientific Conference 5 - 8 Oct 2018 Kuala Lumpur | EARCOS Leadership Conference (ELC) 2018, 25 - 27 Oct 2018, Kuala Lumpur | Annual Conference of the International Federation of Technical Analysts (IFTA) 2018 26 - 28 Oct 2018, Kuala Lumpur | Passive and Low Energy Architecture (PLEA) 2018: Smart and Healthy within the 2-degree Limit Dec 10 - Dec 12 2018, | 17th Asia Pacific Life Insurance Congress, May 30-Jun 01 2019.
Related Associations or Societies: International Federation for Medical and Biological Engineering | European Society for Biomaterials (ESB) | Biomedical Engineering Society (BMES) | American Institute for Medical and Biological Engineering | The European Association of Pharma Biotechnology (EAPB) | The Biotech Research Society | Young European Biotech Network (YEBN) | Japan Bioindustry Association (JBA) | European Federation of Pharmaceutical Industries and Associations (EFPIA)
Track 15: Recent Advances in Bioinformatics, Biomechanics & Biomaterials
Engineering Systems is a science that has played a major role the lives we live in the 21st century. In today’s technology driven world, engineering is the cornerstone and driver of innovation of the devices we utilize daily to improve our quality of life. The latter driver, namely the new ideas, is actually elements of research in engineering. This special section deals with the general topic of long term innovative topics for research in Chemical Engineering, Civilengineering, Electrical engineering, Mechanical engineering, Software engineering, Systems engineering and Interdisciplinary engineering. The peer-reviewed articles will showcase potentially high impact research topics or directions. The objective of this issue is to identify topics that are likely to result in a noteworthy impact on engineering industry in the next 25 to 50 years.
Related Biotechnology Conferences | Bioengineering Meetings
BIO Investor Forum October 17 – October 18, 2018 San Francisco, California | BIO Patient and Health Advocacy Summit October 30 – October 31, 2018 Washington, DC | ICCA activities at IMEX America 2018 October 16 - 18 October 2018 Las Vegas, NV, U.S.A | ICCA activities at IMEX 2019 May 21 - 23 May 2019 | 1st Association of Southeast Asian Prosthodontic Societies and 7th Malaysian Association for Prosthodontic Scientific Conference 5 - 8 Oct 2018 Kuala Lumpur | EARCOS Leadership Conference (ELC) 2018, 25 - 27 Oct 2018, Kuala Lumpur | Annual Conference of the International Federation of Technical Analysts (IFTA) 2018 26 - 28 Oct 2018, Kuala Lumpur | Passive and Low Energy Architecture (PLEA) 2018: Smart and Healthy within the 2-degree Limit Dec 10 - Dec 12 2018, | 17th Asia Pacific Life Insurance Congress, May 30-Jun 01 2019.
Related Associations or Societies: International Federation for Medical and Biological Engineering | European Society for Biomaterials (ESB) | Biomedical Engineering Society (BMES) | American Institute for Medical and Biological Engineering | The European Association of Pharma Biotechnology (EAPB) | The Biotech Research Society | Young European Biotech Network (YEBN) | Japan Bioindustry Association (JBA) | European Federation of Pharmaceutical Industries and Associations (EFPIA)
Global Market Study of Pharmaceutical Biotechnology and Bioengineering
The worldwide market for pharmaceuticals is projected to grow from $1 trillion in 2015 to $1.3 trillion by 2020, with compound annual growth rate of 4.9%. Pharmaceutical and biotechnology industries are significantly investing in R&D in the recent year owing to rising demand for advanced medicines. This may be attributed to increasing aging population, incidence of chronic diseases, and communicable diseases.
The pharmaceutical sector has consistently been one of the most R&D intensive industries in the United States. The research-based industry generally allocates around 15 to 20 percent of revenues to R&D activities and invests over $50 billion on R&D annually. Although the United States remains the global leader in innovative R&D investment, producing more than half the world’s new molecules in the last decade, R&D performed in the United States has become increasingly expensive relative to emerging economies in Asia, such as China and Singapore.
Integrated research ecosystem enables companies to access multidisciplinary capabilities in a single location, which improves R&D decision-making and accelerates drug discovery and development. More than 30 world’s leading biomedical sciences companies (including GlaxoSmithKline, Novartis and Takeda) are leveraging to drive innovation, growing the nation’s biotechnology and pharmaceutical industry by more than 30 percent.
Biomedical engineering involves applying engineering principles to biology, medicine and healthcare to solve problems. It is a fast-growing area of engineering and individuals would typically find themselves working in health services, the medical devices industry/ research. The global medical devices market alone is estimated to be worth USD $381billions, and America accounts for around 45% of the global market.
Some of the major players in the market are
· Thermo Fisher Scientific (US),
· Merck (Germany),
· Horizon Discovery Group (UK),
· GenScript (US),
· Sangamo BioSciences (US),
· Integrated DNA Technologies (US),
· Lonza Group (Switzerland),
· New England Biolabs (US),
· OriGene Technologies (US),
· Transposagen Biopharmaceuticals (US),
· Editas Medicine (US),
· CRISPR Therapeutics (Switzerland).
Biotechnology Market in Different Regions/Continents
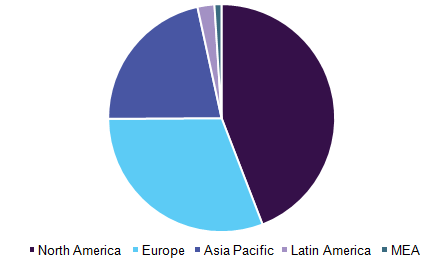 .
.
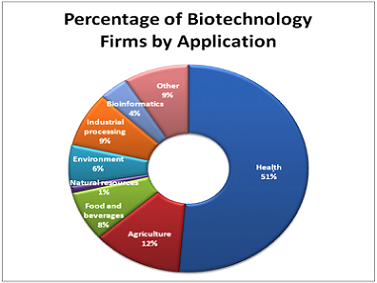
Careers in Biomedical Sciences
We gratefully thank all our wonderful Speakers, Conference Attendees, Students, Media Partners, Associations and Exhibitors for making Pharma Biotech 2017 Conference the best ever!
The 20th International Conference on Pharmaceutical Biotechnology, hosted by the Conference Series LLC Ltd was held during December 07-09, 2017 at Madrid, Spain based on the theme “Widespread Applications and Advancements in Pharmaceutical Biotechnology ". Benevolent response and active participation was received from the Organizing Committee Members along with Scientists, Researchers, Students and leaders from various fields of Pharma Biotech, who made this event a grand success.
Know more at Pharma Biotech 2017 Proceedings
Conference Series LLC Ltd expresses its gratitude to the conference Moderators, namely Dr. Ana Cristina Calvo, for taking up the responsibility to coordinate during the sessions. We are indebted to your support.
The conference was initiated with the Honourable presence of the Keynote forum. The list includes:
- Elena Gómez-Sanz, ETHZ, Switzerland
- Isabel Desgagné-Penix,Université du Québec à Trois-Rivières, Canada
We are grateful to our Speakers of Special session. The list comprises:
- Wolfgang B Fischer, National Yang-Ming University, Taiwan
- Vladimir A Baulin, Universitat Rovira I Virgili, Spain
The meeting reflected various sessions, in which discussions were held on the following major scientific tracks:
- Biopharmaceuticals
- Novel Approaches in Biopharmaceutics
- Nanoparticles in Biopharmaceuticals
- Biotechnology in Health Care
- Formulation of Biotech Products
- Agriculture Biotechnology
- Advances in Biotech Manufacturing
- Biotechnology and its Applications
- Biopharmaceutical Engineering
- Chemical Biotechnology
- Biomass and Bioenergy
- Industrial and Microbial Biotechnology
- Biopharmaceutical Production in Transgenic Animals
- Development on Recombinant Protein
- Nanobiotechnology
- Biotech Companies and Market Analysis
Conference Series LLC Ltd offers its heartfelt appreciation to organizations such as Bentham Science and our esteemed supporters SelectScience, Trade Show Alerts Medgenera, CrowdReviews, PBR Clinical Biometrics, Bio-Equip, Nanobay, Pharma Focus Asia, Times International eindiabusiness and other eminent personalities who supported the conference by promoting in various modes online and offline which helped the conference reach every nook and corner of the globe. Conference Series LLC Ltd also took privilege to felicitate the Keynote Speakers, Organizing Committee Members, Chairs and Exhibitors who supported this event.
>|| Past Conferences Keynote Speakers ||<
We thank all our Honorable Keynote Speakers for their valuable presence, namely
- Elena Gómez-Sanz, ETHZ, Switzerland
- Topolcan Ondrej, Charles University, Czech Republic
- Jianhua Luo, University of Pittsburgh School of Medicine, USA
- Isabel Desgagné-Penix, Université du Québec à Trois-Rivières, Canada
- Vladimir Torchilin, Northeastern University, USA
- Andreas Bernkop-Schnürch, University of Innsbruck, Austria
- Arwyn T Jones, Cardiff University, UK
- Raid Alany, Kingston University, UK
- Olivia Merkel, Ludwig-Maximilians-Universität München, Germany
- Prof. Thomas Prevenslik, (QED Radiations, China)
- Margit Ingeborg Schulze, Bonn-Rhein-Sieg University of Applied Sciences, Germany
- Patrick Fickers, Gembloux Agro BioTech, University of Liege, Belgium
- Volkmar Weissig, Midwestern University, USA
- Csilla Keyges, Solvo Biotechnology, Hungary
- Felix Kratz, CytRx Corporation, Germany
- Amiram Goldblum, The Hebrew University of Jerusalem, Israel
- Joel Richard, IPSEN, France
- Kang Choon Lee, SungKyunKwan University, Republic of South Korea, and more….
>|| Past Conferences Chairs/Co-Chairs ||<
We extend our appreciation towards our Chairs and Co-Chairs of the sessions, namely
- Wancai Yang, University of Illinois at Chicago, USA
- Vladimir A Baulin, Universitat Rovira I Virgili, Spain
- Bodour Salhia, University of Southern California, USA
- Topolcan Ondrej, Charles University, Czech Republic
- Mercedes Piedad de León-Bautista, Central adn, Mexico
- Andreas Bernkop-Schnürch, University of Innsbruck, Austria
- Amiram Goldblum, The Hebrew University of Jerusalem, Israel
- Raid Alany, Kingston University, UK
- Arwyn T Jones, Cardiff University, UK and more…
>|| Past Conferences Moderators ||<
Conference series LLC Ltd expresses its gratitude to the conferences Moderators, for taking up the responsibility to coordinate during the sessions, we are indebted to your support, namely
- Dr. Faiez Alani (McMaster University, Canada)
- Dr. Kristian M Muller (Bielefeld University, Germany)
- Volkmar Weissig, Midwestern University, USA
- Anvita Karara, Carnegie Mellon University, USA
- Elena Landoni, National Cancer Institute, Italy
- Helen Snooks, Swansea University Medical School, UK and more…
>|| Past Conferences Sponsors/Exhibitors ||<
A very special thanks to our Sponsors, Ad-Sponsor and Exhibitors to have bestowed and their faith in collaborating with us to make this event a fruitful one. We hope you continue your support in our future endeavors.
- Bionanonet
- CRS controlled release society
- FIP international Ferderation
- Nanonextnl
- Nanonet
- Pion
- Practicle Work
- Metrics Ltd
- ESCO and more…
>|| Past Conferences Media Partners ||<
Conference series LLC Ltd offers its heartfelt appreciation to organizations such as Bentham Science, Biopharma ASIA, Pharma Vision, Pharmaceutical-Tech.com, Drug Target Review, Labiotech.eu, Pharmaceutical Tech, NIA, The Pharma Review Event listing sites and other eminent personalities who supported the conferences by promoting in various modes online and offline which helped the conferences reach every nook and corner of the globe.
///****////Mark your calendars for the upcoming conference, we are hoping to see you soon!////****////
Conference Highlights
- Biotechnology in Drug Discovery and Development
- Pharmaceutical Biotechnology: Concepts and Applications
- Biopharmaceuticals and Biotherapeutics: Recent Advancements
- Biotech Products Manufacturing: Novel Approaches & Technologies
- Biopharmaceutical Production in Transgenic Animals
- Nanotechnology in Biopharmaceuticals
- Pharmacogenomics & Pharmacogenetics
- Vaccines Manufacturing: Challenges and Solutions
- Recombinant DNA Technology in Pharma and Medicine
- Bioavailability, Bioequivalence & Bioethics
- Biomedical Engineering in Healthcare
- Recent Advances in Diagnostic and Therapeutic Systems
- Medical Devices: Challenges and Applications
- Pharmaceutical & Genetic Engineering
- Recent Advances in Bioinformatics, Biomechanics & Biomaterials
- Training and Certification
- Entrepreneurs Investment Meet
To share your views and research, please click here to register for the Conference.
To Collaborate Scientific Professionals around the World
| Conference Date | March 25-26, 2019 | ||
| Sponsors & Exhibitors |
|
||
| Speaker Opportunity Closed | |||
| Poster Opportunity Closed | Click Here to View | ||
Useful Links
Special Issues
All accepted abstracts will be published in respective Our International Journals.
- Journal of Biotechnology & Biomaterials
- Advances in Genetic Engineering & Biotechnology
- Biology and Medicine
Abstracts will be provided with Digital Object Identifier by

















































